Do the total mercury concentrations detected in fish from Czech ponds represent a risk for consumers? | Scientific Reports - Nature.com
Abstract
Mercury is one of the important pollutants of the environment. Therefore, it's necessary to monitor quantity of mercury especially in aquatic ecosystems. The main goal of the presented study was to compare the content of total mercury in tissues of fish coming from the Czech Republic, an important carp exporter, with focus on comparison of mercury content between 3 different ponds, its comparison between different fish species and between different tissues of the same species, and estimation whether the mercury content in tissues meets the limit given in the Commission Regulation (EC) No. 1881/2006 or not. Total mercury concentration was measured in 90 fish specimen sampled from three ponds (Velky Kocelovicky, Mysliv and Zehunsky) in autumn 2018. The values of total mercury in fish tissues was measured by atomic absorption spectrometry. The content of total mercury in the tissues decreased as follows: muscle > liver > gonads > scales. The highest average content of total mercury in muscle was 0.1517 ± 0.0176 mg/kg coming from pike caught in Velky Kocelovicky pond. In contrast, the lowest average content of total mercury in muscle 0.0036 ± 0.0003 mg/kg was found in carp tissue coming from the locality of Zehunsky pond. We confirmed that the predatory fish are more exposed to mercury than non-predatory fish. None of the monitored localities exceeded the set regulatory limit. Thus, our study shows that fish coming from these ponds are safe in terms of total mercury content.
Introduction
The aquatic environment contains a high amount of contaminants that can pose a risk to the human population. Mercury (Hg) is one of the most widespread and ubiquitous contaminants in aquatic ecosystems, mainly due to its global distribution through multiple pathways (air, water, and food)1. Mercury gets into the aquatic environment naturally or due to human activities2,3,4,5. The most important sources of mercury in the aquatic environment are erosion, emissions from industry, mining and combustion of fossil fuels6,7,8,9. Mercury has the ability to bioaccumulate, its concentration increases in the food chain, so a higher content of mercury is found in predatory fish2,10,11,12,13. The concentration of mercury in fish depends mainly on the concentration of mercury in the aquatic environment14 where, due to microorganisms contained in the bottom sediment, inorganic mercury is converted into a more available organic form called methyl mercury (MeHg) which enters the body of the fish through the food chain12,15. Mercury concentrations are also affected by the age and weight of fish16,17.
As a result, the accumulation of methyl mercury in aquatic organisms poses a risk not only for humans but also for wildlife13,18. In order to prevent risk connected to environmental mercury exposure, Minamata convention on mercury was signed by more than 120 countries including the Czech Republic and came into force in 2017. As a result, since 2020 the manufacture, import and export of mercury-added products, with certain exceptions, has no longer been allowed19.
Aquaculture has been historically a significant activity in the Czech Republic with common carp being the most dominant species. In 2017, the aquaculture production of the Czech Republic was 21,685 tons, out of these 85.1% was common carp20. Main part of the domestic production is intended for export21. Surface waters represent a reservoir of numerous environmental contaminants. Mercury and other heavy metals are able to accumulate in aquatic organisms, especially fish. Since the Czech Republic is a landlocked country having no access to ocean, freshwater aquaculture is of a high importance there. Fish pond farming, a traditional form of aquaculture in the Czech Republic, is considered as its national heritage22.
Human exposure to mercury occurs mainly through the consumption of aquatic animals, especially fish, which come from a contaminated environment and are consumed frequently and for a long time23. Fish form an integral part of the diet of a large number of people, so it is necessary to monitor the mercury content, as the highest concentrations of mercury can be found in the muscle of fish14,24. The mercury content in fish muscle is regulated by Commission Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. The regulation distinguishes between two groups of fish. While the maximum level of 0.5 mg/kg (wet weight) has been set for freshwater fish with the exception of pike (Esox lucius), eel (Anguilla spp.) and sturgeon (Acipenser spp.), up to 1 mg/kg (wet weight) of mercury can be detected in marine fish and pike, eel and sturgeon. The limits in the regulation are expressed as total mercury (THg). However, MeHg is primarily responsible for bioaccumulation in the muscle tissue of fish with a MeHg fraction of 83–90% of the-total mercury concentration25. Therefore, World Health Organization has set provisional tolerable weekly intake for MeHg to be 1.6 µg/ kg human body weight/week26.
The aim of the present paper was to analyze and present the results of research on mercury content in tissues of fish coming from the Czech Republic, an important carp exporter, with focus on comparison of mercury content between 3 different ponds, comparison of mercury content between different fish species and between different tissues of the same species, and estimation whether the mercury content in tissues meets the limit given in the Commission Regulation (EC) No. 1881/2006 or not.
Materials and methods
To evaluate the content of THg, 3 ponds were selected: Zehunsky, Mysliv and Velky Kocelovicky. The location of the ponds is shown in Fig. 1. The Zehunsky pond (Chlumec nad Cidlinou fishing) has an area of approximately 258 ha. Mysliv pond (Klatovy fishing) has an area of 60 ha. The Velky Kocelovicky pond (Lnare fishing) has an area of 33 ha. The fish stock in these ponds is composed primarily of common carp (Cyprinus carpio) and secondary species of predatory fish. These fish are represented by catfish (Silurus glanis), pike (Esox lucius) and zander (Sander lucioperca). These three ponds were selected because of their high yield of fish intended for human consumption. Mysliv pond is located in West Bohemia and Velky Kocelovicky in the South Bohemian region, which is considered the most important terms of aquaculture. The Zehunsky pond is located in the Central Bohemian region and is the largest pond there. The fish were caught during the autumn season together with fish for trading.
Location of the sampling sites: red—Zehunsky pond, yellow—Mysliv pond, green—Velky Kocelovicky pond.
The fish were killed by a blunt blow to the head, followed by an overcut of the gill arch to bleed. The fish were weighed and the total body length was measured. The characteristics of the analyzed fish are given in Table 1. Muscle, liver, gonad and scale samples were taken from these fish during autopsy. The samples were stored at − 20 °C until analysis. Scales were also samples to determine the age of the fish.
The age of the fish was determined by visual inspection of scales with a help of binocular magnification instrument Meoflex RI 21P (Meopta, Czech Republic) with the exception of catfish, where the age was not determined due to the absence of scales.
The content of THg was determined on a single-atomic absorption spectrometer AMA 254 (Altec Ltd., Czech Republic), which allows determination of THg without sample pre-treatment. The accuracy of the results was verified using standard reference material (CRM No. 13 HUMAN HAIR and BCR-CRM 464, Tuna, IRMM, Belgium). The limit of quantification (LOQ) was 0.001 mg/kg. The THg is expressed in mg/kg fresh weight of analyzed tissues.
The THg liver/muscle ratio is the ratio of liver to muscle Hg concentrations and is calculated as follows: [THg in liver (mg/kg)/THg in muscle (mg/kg)]. THg liver/muscle ratio was calculated from the arithmetic mean.
Hazard index was calculated on the basis of the Kannan et al.27 method. The hazard index (HI) for mercury is ratio dose (D) to the upper level of daily mercury intake during a lifetime estimated without toxic effects (RfD). The formula for this calculation is as follows: HI = D/RfD. The estimated dose (D) can be calculated as D = C × I/W × 1000 where C = concentration of mercury in fish (µg/g), I = ingestion rate of fish (g/day), W = average body weight (70 kg). RfD value for mercury is 1 × 10–4 mg/kg/day28. In the Czech Republic, annual consumption of freshwater fish is 1.3 kg per person29. If the hazard index is less than 1, the mercury exposure could be regarded as unlikely to lead to adverse health effects.
Statistical analysis
A total of 10 individuals of each fish species from each pond were analyzed. The age of fish between ponds was compared by one-way ANOVA (carp, pike) and t-test (zander). The mercury content in muscle, liver, gonads and scales of each fish was measured. Factorial ANOVA followed by the Tuckey post hoc test were used to assess the effect of sampling site, fish species and fish tissue on THg. The same test was used to analyse differences in mercury content in gonads between males and females taking into account sampling site and fish species.
P < 0.05 was considered significant in all tests. Statistical analyses were performed using Statistica, version 13 (TIBCO Software Inc.).
Ethical statement
All the methods used in this study followed relevant guidelines and regulations, in particular Act No. 246/1992 Coll., on the Protection of Animals against Cruelty, as amended and Decree No. 419/2012 Coll., on the Protection, Breeding and use of Experimental Animals, as amended. Moreover, the competent authority (Ethical Committee for Protection of Animals in Research of the University of Veterinary Sciences Brno) approved the fish sampling and protocols of the present study and reporting herein follows the recommendations in the ARRIVE guidelines.
Results
The age of the analyzed fish ranged from 3.0 to 4.2 years. Higher age of carp was found in the Zehunsky and Velky Kocelovicky pond, lower in the Mysliv pond (Table 1). The difference was significant (ANOVA, F(2,26) = 6.408, P = 0.005). The age of pike did not differ significantly between ponds (ANOVA, F(2,27) = 0.300, P = 0.743), the same applies to zander (t-test, t(18) = 1.809, P = 0.087).
Fish used for the determination of THg were caught in the ponds Velky Kocelovicky, Zehunsky and Mysliv.
THg in fish differed significantly between studied ponds, fish species and tissue (factorial ANOVA, effect of studied pond: F(2,323) = 20.468, P < 0.001; effect of fish species: F(3,323) = 41.243, P < 0.001; effect of tissue: F(3,323) = 120,591, P < 0.001; effect of interaction of all three predictors: F(18,323) = 5.815, P < 0.001).
The highest content of THg in fish from Velky Kocelovicky pond was observed in the muscle of pike (0.1517 ± 0.0176 mg/kg), the lowest content was determined in samples of scales and gonads of carp (0.0010 ± 0.0001 and 0.0012 ± 0.0001 mg/kg). In the Mysliv pond, the highest content of mercury was measured in the muscle of pike (0.1299 ± 0.0103 mg/kg), whereas the lowest values were observed in the scales and gonads of common carp (0.0013 ± 0.0001 and 0.0014 ± 0.0001 mg/kg). The situation was the same in Zehunsky pond; the highest content of mercury was measured in the muscle of pike (0.0398 ± 0.0108 mg/kg) and the lowest values were determined in the gonads of common carp (0.0006 ± 0.0000 mg/kg) and in the scales of carp and pike (0.0007 ± 0.0001 and 0.0012 ± 0.0001 mg/kg) (Table 2, Fig. 2).
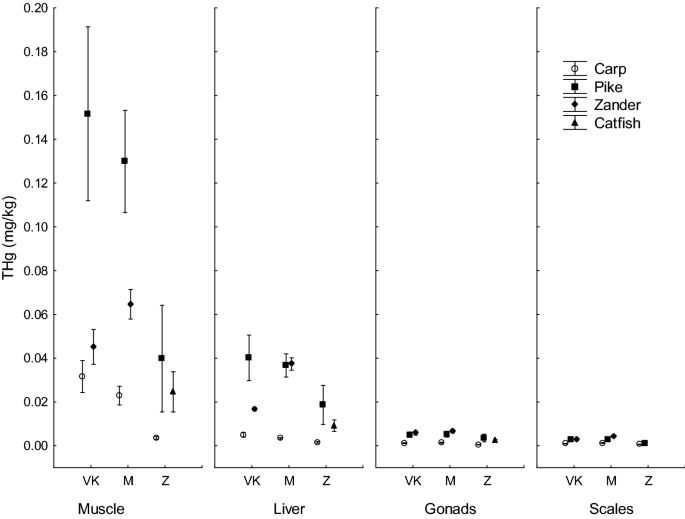
Total mercury content (mg/kg wet weight) in fish tissue of carp, pike, zander and catfich caught in three sampling sites: Velky Kocelovicky (VK), Mysliv (M) and Zehunsky (Z). Vertical bars denote 0.95 confidence intervals.
THg content in tissues decreased in the order: muscle > liver > gonads > scales. Differences between tissues were significant (P < 0.05) for pike, zander and catfish in all ponds. For carp, the THg in different tissues did not differ significantly (P > 0.05) in any pond (Table 2).
In the locality Velky Kocelovicky pond, a statistically significant difference (P < 0.05) was found in the content of THg in the muscle of pike and the other two species (common carp, zander). No significant difference (P > 0.05) was found in THg content in the muscle of common carp and zander. Considering the Mysliv pond, a statistically significant difference (P < 0.05) was found between all three species, being the highest in pike and the lowest in carp. In the Zehunsky pond, no significant interspecific differences (P > 0.05) were found in the mercury content in fish muscle (Table 2).
The THg content in the muscle of carp varied from 0.0036 ± 0.0003 mg/kg in Zehunsky pond to 0.0316 ± 0.0032 mg/kg in Velky Kocelovicky and did not differ significantly between the three ponds (P > 0.05). Similarly, in zander the mercury content in muscle did not differ (P > 0.05) between Velky Kocelovicky (0.0452 ± 0.0035 mg/kg) and Mysliv (0.0647 ± 0.0030 mg/kg). The situation was different for pike, where the mercury content in the muscle ranged from 0.0398 ± 0.0108 mg/kg in Zehunsky to 0.1517 ± 0.0176 mg/kg in Velky Kocelovicky and differed significantly (P < 0.05) (Table 2).
The mercury content in the liver of fish ranged from 0.0016 ± 0.0002 mg/kg (carp, Zehunsky pond) to 0.0402 ± 0.0046 mg/kg (pike, Velky Kocelovicky) and did not differ significantly either between species or sampling sites (Table 2).
In scales, the lowest mercury content was 0.0007 ± 0.0001 mg/kg (carp, Zehunsky) and the highest 0.0042 ± 0.0002 mg/kg (zander, Mysliv). The mercury content in scales did not differ significantly either between fish species or sampling sites (Table 2).
The mercury content in the gonads ranged from 0.0006 ± 0.0000 mg/kg (carp, Zehunsky pond) to 0.0067 ± 0.0003 mg/kg (zander, Mysliv) and did not differ significantly either between species or sampling sites (Table 2).
A detailed analysis of the mercury content in the gonads of male and female fish found values from 0.0006 ± 0.0001 mg/kg and 0.0006 ± 0.0000 mg/kg, respectively (male and female, carp, Zehunsky pond) to 0.0079 ± 0.0002 (male, zander, Mysliv) (Table 3, Fig. 3). Factorial ANOVA used to identify differences in mercury content in gonads between males and females and taking into account sampling site and fish species did not find significant differences between males and females (effect of gender in factorial ANOVA: F(1,77) = 2.839, P = 0.096). Despite this, the Tuckey post hoc test revealed differences in the mercury content in the gonads of males and females of pike from the Mysliv pond; values were higher in males (Table 3).
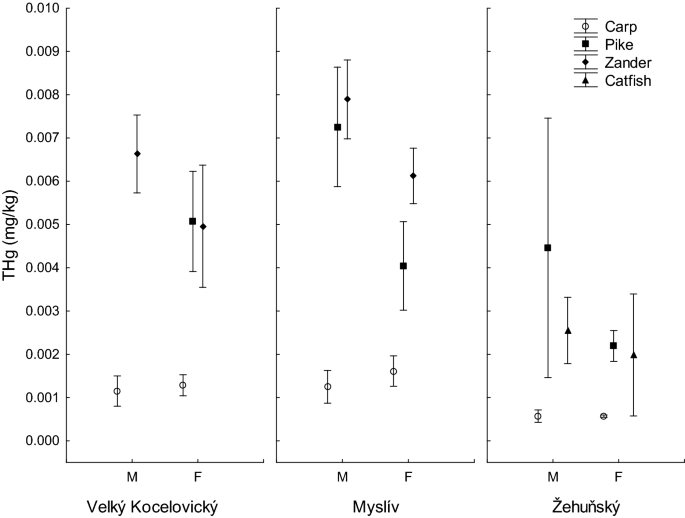
Total mercury content (mg/kg wet weight) in gonads of male (M) and female (F) carp, pike, zander and catfich caught in three sampling sites. Vertical bars denote 0.95 confidence intervals.
THg liver/muscle ratios was calculated for fish from individual ponds. Ratio higher than 1 means very contaminated location. If ratio is lower than 1 it is insignificant contamination. As these values are far below 1, they indicate that the fish we monitored came from uncontaminated sites. The highest THg liver/muscle ratio was calculated for catfish from Mysliv pond (Table 4).
Hazard index (HI) was calculated for every fish species from three ponds. The highest hazard index was found for pike from the pond Velky Kocelovicky 0.078 and subsequently for pike from the pond Mysliv 0.067. The lowest HI was found in carp 0.002 from pond Zehunsky (Table 4).
Discussion
Mercury content in fish depends on several factors. The first one and one of the most important factors represent the environmental contamination. Generally higher THg concentrations can be expected in fish from areas with a high industrial load, in the proximity of big cities, especially downstream from possible sources of contamination30,31,32. From this viewpoint, low THg concentrations detected in fish from our study are not surprising, as all the 3 breeding ponds were located in countryside far from any industrial plants. Comparable THg concentrations were previously found in common carp cultured in 10 other Czech uncontaminated fishponds24, two different farms/ponds in Austria13, Shadegan International Wetland located in Iran23 and also in carp from other freshwater systems33,34,35. For instance, Vicarova et al.35 monitored the contamination of three water reservoirs in the Bohemian-Moravian Highlands. While THg concentrations detected in carp muscle ranged from 0.0189 to 0.0724 mg/kg, up to 0.0189 mg/kg of mercury was found in liver35. These values correspond well to results of our study.
Due to concerns about possible adverse effects of mercury on fish and also humans consuming them, extensive legislative measures regulating industrial use of mercury have been adopted. All of these measures can potentially lead to the decrease in THg concentrations detected in fish as well as other aquatic organisms. In fact, this was already proven in our previous study investigating spatial and temporal trends in contamination of the Czech part of Elbe River30. Overall long-term decline in THg concentrations of at least 20% during 1965–2012 was also observed in Swedish freshwater fish36.
The age of fish is another factor affecting THg concentrations detected in fish tissues as it is expected that the bioaccumulation of this heavy metal proceeds throughout the whole life of fish. A significant positive correlation between the age of fish and the THg concentrations detected in their muscles was previously found in chub (Leuciscus cephalus) from Morava River basin37. Age-related differences in THg concentrations detected in fish tissues were also reported by Dusek et al.38 and Marsalek et al.39. Although we intended to investigate the fish of similar age, statistical analysis of our data revealed that there was a small but still significant difference in the age of carp from Mysliv pond compared to those caught in Velky Kocelovicky or Zehunsky ponds. This should be taken into account when interpreting data.
Feeding habits of fish affect bioaccumulation of mercury as well. Compared to herbivores, limnivores or omnivores, relatively higher mercury levels were previously detected in predatory species of fish representing a higher trophic level2,10,11,12,40. This is in the full agreement with our study, as generally lower concentrations of mercury were detected in the common carp (omnivorous fish), compared to 3 other carnivorous species (pike, zander, catfish). The significant differences in THg concentrations in fish muscle were revealed between common carp and pike sampled from Velky Kocelovicky and Mysliv pond.
According to some authors, fish sex can also potentially affect bioaccumulation of mercury. By examining possible relationships between the sex of perch (Perca fluviatilis) and mercury accumulation in their gonads, Jankovska et al.41 observed that male gonads (milt) can accumulate higher levels of mercury than do female spawn, probably due to their different compositions41. Other authors focused on differences in THg concentrations detected in muscle tissue between both female and male fish. While female European catfish (Silurus glanis) from Italian rivers displayed higher concentrations of mercury compared to male specimens32, no significant differences between both sexes were revealed in fish from Ebro River (Spain)42. In our study, THg concentrations detected in fish gonads did not significantly differ between both sexes of fish, excepting the pike from Mysliv pond, where significantly higher THg concentrations were observed in male fish compared to those found in females.
A distribution of mercury in fish tissues has been considered as a good indicator of environmental contamination by this heavy metal, since it was reported, that the deposition of mercury in fish differs between uncontaminated and heavily contaminated sites. While liver represents the target organ for mercury accumulation in fish from highly contaminated localities, in the case of lightly contaminated (or uncontaminated) sites, the majority of mercury is being deposited into fish muscle43,44. THg concentrations which we detected in various fish tissues decreased in the following order: muscle > liver > gonads > scales. This is in a good agreement with previous study by Kensova et al.45. Similar results of tissue distribution of mercury with more contaminant detected in carp muscle compared to liver were also obtained by Zhang et al.14. However, these authors did not measure THg concentrations in gonads and scales as we did, but chose intestine and gills instead14.
One of the aims of our study was to compare THg concentrations detected in fish from the three ponds to limits on Hg content stated by current food legislation, especially the Commission Regulation 1881/2006 setting maximum levels for certain contaminants in foodstuffs. According to this legal act, up to 0.5 mg/kg of mercury can be present in muscle of fish and fishery products marketed in the EU, unless they belong to certain predatory species listed in the section of 3.3.2 of the Annex I of this regulation. Since pike has been listed in this section, a maximum level of mercury of 1.0 mg/kg is relevant for this species46. Regardless of that, THg concentrations detected in all three ponds (Mysliv, Zehunsky, Velky Kocelovicky) were substantially lower than limit values. This suggests that the consumption of fish from the ponds does not represent health risks for consumers.
Not only on the basis of the limits set by legislation, but also the results of the hazard index show that fish come from safe locations in term of Hg contamination. The highest hazard index was found in predatory fish especially in pike (0.078 Velky Kocelovicky pond; 0.067 Mysliv pond). The lowest hazard index as expected was calculated in carp (0.002 Zehunsky pond, 0.012 Mysliv pond; 0.016 Velky Kocelovicky pond). It follows that the values of the hazard index are far from 1 and therefore the consumption of fish from these ponds is safe. Novotna et al.30 set the values of the hazard index for fish from the river Elbe, which flows through the Czech Republic. Their hazard index results for fish from this locality are also below 1, therefore no negative impact on the health of people consuming fish from this river is expected.
Conclusion
Mercury content in fish did not exceed legislative limits in any of the three monitored production ponds. Our study shows that farmed fish production in ponds are safe for human consumption in terms of THg concentration. In the Czech Republic, the most commonly consumed fish in open waters is common carp. For common carp, the measured values of THg were at a very low level. We also confirmed in the study that a higher content of mercury content is found in predatory fish, which in our case were pike, zander and catfish.
References
- 1.
Stein, E. D., Cohen, Y. & Winer, A. M. Environmental distribution and transformation of mercury compounds. Crit. Rev. Environ. Sci. Technol. 26, 1–43 (1996).
- 2.
Ciccarelli, C. et al. Assessment of sampling methods about level of mercury in fish. Ital. J. Food Saf. 8, 153–157 (2019).
- 3.
Ditri, F. M. Mercury contamination: What we have learned since Minamata. Environ. Monit. Assess. 19, 165–182 (1991).
- 4.
Monteiro, L. R. & Furness, R. W. Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut. 80, 851–870 (1995).
- 5.
Pitter, P. In Hydrochemie 5th edn (ed. Pitter, P.) (VSCHT Praha, 2015).
- 6.
Hylander, L. D. & Meili, M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci. Total. Environ. 304, 13–27 (2003).
- 7.
Pacyna, E. G. et al. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 44, 2487–2499 (2010).
- 8.
Pai, P., Niemi, D. & Powers, B. A North American inventory of anthropogenic mercury emissions. Fuel Process. Technol. 65, 101–115 (2000).
- 9.
Wang, Q. R., Kim, D., Dionysiou, D. D., Sorial, G. A. & Timberlake, D. Sources and remediation for mercury contamination in aquatic systems: A literature review. Environ. Pollut. 131, 323–336 (2004).
- 10.
Buck, D. G. et al. A global-scale assessment of fish mercury concentrations and the identification of biological hotspots. Sci. Total Environ. 687, 956–966 (2019).
- 11.
Gentes, S. et al. Application of European water framework directive: Identification reference sites and bioindicator fish species for mercury in tropical freshwater ecosystems (French Guiana). Ecol. Indic. 106, 105468. https://doi.org/10.1016/j.ecolind.2019.105468 (2019).
- 12.
Thomas, S. M. et al. Climate and landscape conditions indirectly affect fish mercury levels by altering lake water chemistry and fish size. Environ. Res. 188, 109750. https://doi.org/10.1016/j.envres.2020.109750 (2020).
- 13.
Zupo, V. et al. Mercury accumulation in freshwater and marine fish from the wild and from aquaculture ponds. Environ. Pollut. 255, 112975. https://doi.org/10.1016/j.envpol.2019.112975 (2019).
- 14.
Zhang, J. L. et al. Health risk assessment of heavy metals in Cyprinus carpio (Cyprinidae) from the upper Mekong river. Environ. Sci. Pollut. Res. 26, 9490–9499 (2019).
- 15.
Cerna, M. Opatreni mezinarodnich instituci a Ceske republiky k omezovani rizika znecistovani zivotniho prostredi rtuti. Chem. Listy. 98, 916–921 (2004) ((Article in Czech)).
- 16.
Janouskova, D. & Svehla, J. Mercury concentrations in fish tissues in the water reservoir Rimov, South Bohemia. Crop Sci. 19, 43–48 (2002).
- 17.
Purba, J. S., Silalahi, J. & Haro, G. Analysis of mercury in fish, North Sumatera, Indonesia by atomic absorption spectrophotometer. Asian J. Pharm. 8, 21–25 (2020).
- 18.
Willacker, J. J., Eagles-Smith, C. A. & Blazer, V. S. Mercury bioaccumulation in freshwater fishes of the Chesapeake Bay watershed. Ecotoxicology 29, 459484 (2020).
- 19.
Regulation (EU) 2017/852 of European Parliament and of the council of 17 May 2017 on mercury, and repealing Regulation (EC) No 1102/2008. Official Journal of the European Union.
- 20.
European Commission. The EU Fish Market. https://www.eumofa.eu/documents/20178/415635/EN_The+EU+fish+market_2020.pdf (2020).
- 21.
Nebesky, V., Policar, T., Blecha, M., Kristan, J. & Svacina, P. Trends in import and export of fishery products in the Czech Republic during 2010–2015. Aquacult. Int. 24, 1657–1668 (2016).
- 22.
FAO. Fisheries & Aquaculture—National Aquaculture Sector Overview—Czech Republic. http://www.fao.org/fishery/countrysector/naso_czechrepublic/en (accessed April 24 April 2021) (2021).
- 23.
Rakmanikhah, Z., Esmaili-Sari, A. & Bahramifar, N. Total mercury and methylmercury concentrations in native and invasive fish species in Shadegan International Wetland, Iran, and health risk assessment. Environ. Sci. Pollut. Res. 27, 6765–6773 (2020).
- 24.
Celechovska, O., Svobodova, Z., Zlabek, V. & Macharackova, B. Distribution of metals in tissues of the common carp (Cyprinus carpio L.). Acta Vet. Brno 76, 93–100 (2007).
- 25.
Cerveny, D. et al. Fish fin-clips as non-lethal approach for biomonitoring of mercury contamination in aquatic environments and human health risk assessment. Chemosphere 163, 290–295 (2016).
- 26.
WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). https://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx.
- 27.
Kannan, K. et al. Distribution of total mercury and methyl mercury in water, sediment, and fish from south Florida estuaries. Arch. Environ. Con. Tox. 34, 109–118 (1998).
- 28.
US EPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories Documents. Volume 2: Risk Assessment and Fish Consumption Limits, Third Edition. https://www.epa.gov/fish-tech/guidance-assessing-chemical-contaminant-data-use-fish-advisories-documents (accessed 8 May 2021) (2000).
- 29.
Ministry of Agriculture of the Czech Republic. Situacni a vyhledova zprava—Ryby. http://eagri.cz/public/web/file/666957/Ryby_2020_web.pdf (accessed 8 May 2021, in Czech) (2020).
- 30.
Novotna, K., Svobodova, Z., Harustiakova, D. & Mikula, P. Spatial and temporal trends in contamination of the Czech part of the Elbe River by mercury between 1991 and 2016. Bull. Environ. Contam. Toxicol. 105, 750–757 (2020).
- 31.
Raldua, D., Diez, S., Bayona, J. M. & Barcelo, D. Mercury levels and liver pathology in feral fish living in the vicinity of a mercury cell chlor-alkali factory. Chemosphere 66, 1217–1225 (2007).
- 32.
Squadrone, S. et al. Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian rivers. Chemosphere 90, 358–365 (2013).
- 33.
Cerveny, D. et al. Contamination of fish in important fishing grounds ...
Comments
Post a Comment