An ancient link between heart and head — as seen in the blobby, headless sea squirt - Nature.com
The head is stately, calm, and wise,
And bears a princely part;
And down below in secret lies
The warm, impulsive heart. — John Godfrey Saxe, 1898
For centuries, writers have mused on the heart as the core of humanity's passion, its morals, its valour. The head, by contrast, was the seat of cold, hard rationality. In 1898, US poet John Godfrey Saxe wrote of such differences, but concluded his verses arguing that the heart and head are interdependent. "Each is best when both unite," he wrote. "What were the heat without the light?" At that time, however, Saxe could not have known that the head and the heart share a deep biological connection.
In the past 15 years, scientists have uncovered a developmental link between the two. In 2010, for example, researchers revealed that the same small pool of cells that divides and differentiates to form the heart in mouse embryos also gives way to muscles in their throats and lower heads1. Key components of the two are cut from the same cloth.
Even more surprising is that the embryonic head–heart connection pre-dates the evolutionary origin of vertebrates, and perhaps even of the head itself. Researchers stumbled on the link while studying sea squirts, blobby, sedentary marine creatures, found affixed to the sea floor, that have two openings — one for sucking water in and the other for squirting it out — hence the name.
Sea squirts belong to a group of spineless animals, called tunicates, that are vertebrates' closest living relatives, despite their many differences. And even though tunicates lack a true head, the evolutionary link between the head and heart is strong in them. "When I first heard about this, I was like, what are you talking about?" says Billie Swalla, an evolutionary developmental biologist at the University of Washington in Seattle, with a laugh.
Tiny feather wing beetle reveals new way to fly
Researchers have identified a group of cells in sea-squirt embryos that divide and differentiate to form both their hearts and the muscles in a structure similar to part of the vertebrate throat. This suggests that a head–heart connection arose before those two lineages diverged from one another hundreds of millions of years ago. Now, Swalla and other researchers are trying to determine how far back the link goes, and whether it arose in an immobile, headless animal, similar to the sea squirt, or one that faced forward as it swam, with a head evolving along with its heart. "That's the question that keeps me up at night — that and COVID," Swalla says.
Looking for links
Most animals have a heart, in the vaguest sense of the word, meaning any vessel that pumps fluid around a body. But in vertebrates, the heart is a distinct, layered organ with parts for inflow and outflow that keep a rhythm. Until about a decade ago, the evolutionary origin of the chambered heart was mysterious, because similar forms of the organ weren't apparent in animals previously thought to be vertebrates' closest kin: the slim, mobile lancelet. These translucent, arrow-like animals have such a rudimentary circulatory system — a simple pumping tube — that purists consider them heartless. A link seemed to have been lost to time.
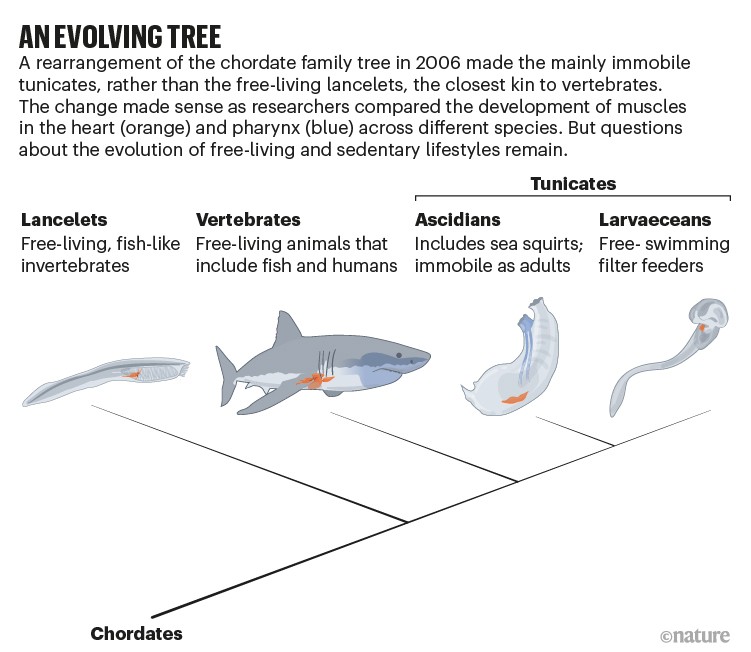
Curiosity was reignited in 2006, when a study suggested that lancelets were not, in fact, the closest living relative to vertebrates2. This analysis, based on genetic analyses of dozens of chordates, the phylum that includes vertebrates, lancelets and tunicates, found that the closest relations are actually the tunicates (see 'An evolving tree'). The rearrangement initially shocked many researchers because, whereas lancelets vaguely resemble fish, sea squirts look more like a large wad of gum — from the outside, at least. "Tunicates should therefore no longer be considered as 'primitive'," the authors concluded.
Marine biologists who had examined the anatomy of tunicates were somewhat relieved by this result, however. Linda Holland, an evolutionary developmental biologist at the Scripps Institution of Oceanography at the University of California, San Diego, says that hypothetical scenarios about how the chordate body had evolved over evolutionary time "just didn't make sense" with the earlier family tree. For example, the lancelet heart looks nothing like those of vertebrates, whereas the sea squirt's bears certain similarities, and is sophisticated.
Beneath the tunicate's unassuming exterior lies a layered, V-shaped heart surrounded by a spiral of muscle fibres that contract the organ in a gradual, wringing motion that keeps fluid flowing in one direction. The heart can also reverse the direction of flow.
Heart cells on the move
To explore the evolution of the tunicate heart, Bradley Davidson, a postdoctoral researcher at the University of California, Berkeley, in the early 2000s, set his sights on the sea-squirt species Ciona intestinalis. He followed in a tradition of developmental biologists who, for more a century, had painstakingly tracked the division and migration of individual cells in embryos. Davidson genetically manipulated sea-squirt embryos so that cells that express a gene involved in vertebrate heart formation, Mesp, would glow green under fluorescent light. When he looked at the embryos under a powerful microscope, a clump of around 16 cells that would form the heart at later stages of development dutifully lit up.
In 2005, another postdoctoral researcher, Lionel Christiaen, joined the Berkeley lab, and decided to repeat Davidson's experiment with Mesp. He photographed the embryos with the glowing clump of cells one morning, and then left campus. For no particular reason, he decided to return later that evening and check on them again. "It was a day when I was hungry for science," recalls Christiaen, now an evolutionary biologist at the Sars International Center for Marine Molecular Biology at the University of Bergen in Norway.

How warp-speed evolution is transforming ecology
To his surprise, some of the glowing green cells had migrated to the opposite side of the embryos, where they formed a ring near the animal's developing pharynx. In adult sea squirts, the pharynx fills with water, filters out plankton to the digestive system and releases the rest for the animal to squirt back out. In vertebrates, the pharynx is part of the throat. Fish use theirs to process gulps of water from their open mouths. Christaen was aware of work suggesting that muscles in the mouse heart derived from the same pool of embryonic cells as some in the lower head3. He wondered if he might be observing the same thing in tunicate embryos.
Shared heart–head precursors were not known back in 1983, when biologists studying vertebrate origins put forward the 'new head' theory4 to describe the evolution of a distinct head with a thumping heart positioned just below. These authors depicted a scenario in which natural selection drove a cascade of features that improved the ability of vertebrates to eat and hunt. One such adaptation was the evolution of jaws from the neural-crest cells that give rise to the vertebrate skeleton. Other key adaptations would have included refined sense organs in the hunter's new head, and a complex brain to integrate the signals.
The authors of this theory focused mostly on the cells that created jaws and other bones. They left open the evolution of muscles around the pharynx and lower head that would make jaws operable. The evolution of those muscles remained elusive for decades, until Christiaen found a clue in that ring of cells in the tunicate embryos with activated Mesp.
Together with his colleagues, Christiaen ran a second experiment. This time, they made cells that express genes related to the formation of the lower vertebrate jaw — Islet and Tbx1/10 — glow green in tunicate embryos. Sure enough, these cells derived from the same pool of cells that created the heart. In a 2010 report5, the team coined the term 'cardiopharyngeal' to describe embryonic cells that form the heart and pharynx in tunicates, and they suggested that these cells were probably present in a shared ancestor of tunicates and vertebrates. Furthermore, they said these special cells would have been instrumental in the coevolution of the circulatory, respiratory and feeding system of vertebrates. "The cardiopharyngeal cells preceded the emergence of the jaw," Christiaen says. "They were there ready to welcome head bones with muscles."
Have heart, won't travel
Another evolutionary biologist studying the origin of vertebrates and their kin, Cristian Cañestro, at the University of Barcelona in Spain, says that Christiaen's study suggested that "the machinery that made a 'new head' was present in the last common ancestor" of vertebrates and tunicates. But did those ancestors actually have a head?
It is possible that they did, and that tunicates lost theirs. Two facts support this idea. Before they affix themselves to a rock or a bit of the sea floor, tunicate larvae are mobile, free-swimming creatures, with a definable front and tail. And one type of tunicate, a small group named larvaceans — because they superficially look like swimming larvae — remain mobile their entire lives. Less than few centimetres long, larvaceans fumble through the ocean wiggling their tails. That motion generates a water current that flows through a bubble-like 'house' they generate around their bodies, allowing them filter out plankton.

Australia's cane toads evolved as cannibals with frightening speed
For some researchers, larvaceans' existence seemed to point in favour of a travelling tunicate ancestor. Cañestro decided it was time to learn more about the creatures, so he started a colony of them in the basement of his lab in Barcelona. Kept in a glass tank of circulating salt water, hundreds of larvaceans hover like dust for the duration of their fleeting lifespan. "On day five," Cañestro explains, "they explode, releasing hundreds of sperm or eggs that float at the surface of the water."
When his graduate student, Alfonso Ferrández, was searching for a doctoral project, Cañestro took him into the basement to see the larvaceans, and suggested he study the development of their hearts. Ferrández began by fishing for Mesp and other genes known to control heart development in tunicates and vertebrates. But couldn't find them. He played with protocols. He repeated experiments. He deployed a variety of computer programs to sift through genomic sequences. All to no avail. Finally, he and Cañestro concluded that most of the heart genes simply were not there. Missing genes wouldn't have been very surprising in the larvaceans — they have one of the smallest genomes in the animal kingdom — but it was curious, because they still have a beating heart and plenty of other body parts that the genes encode in other animals. "That's when I started to be very into this project," says Ferrández. "I wasn't in love with the heart story when I started, but this was interesting."
That enthusiasm kept Ferrández going as COVID-19 swept around the globe. Even during Spain's strict lockdown, he got permission to go to the lab to keep the larvaceans alive, as he plugged away at his project. Although Mesp and other known genes were missing, he managed to recover a few genes that contributed to the formation of the organism's simple heart, which comprises just eight muscle cells that rhythmically contract to pump fluid around the body. Over evolutionary time, larvaceans managed to survive gene loss, finding other ways to build body parts that allowed them to move, eat and procreate, says Cañestro. "Gene losses can be adaptive," he says.

Sponge cells hint at origins of nervous system
Rather than representing an ancestral condition, the larvaceans seem to be strange offshoots of the tunicate family, the Barcelona team concluded in a paper6 published last November. With that being the case, the researchers suggested that the ancestor of tunicates was probably immobile like sea squirts rather than free-living like the highly modified larvaceans. "I love this paper," says Davidson, now at Swarthmore College in Pennsylvania. To him, the work showcases how diversity arises in evolution from the same starting blocks.
But in all that diversity, a trail that traces back animals' ancestry is hard to spot. Despite the latest larvacean study, Holland is unconvinced that tunicates were originally immobile. To support her argument, she lists reasons why sea squirts could be outliers, and names features that larvaceans share with lancelets, suggesting that they retain ties to an earlier state of being.
In the coming years, she and other researchers will continue to uncover details on how vertebrates and their closest kin develop, cell by cell, gene by gene, and they will learn what genomic tweaks occurred over hundreds of millions of years to make it all possible. The only seemingly sure thing at the moment is that these changes took place to the thump of a beating heart.
"The vertebrate heart probably evolved from something akin to a tunicate heart, but remember — tunicates are evolving rapidly," Holland muses. "We'll never know for sure what their common ancestor was like, except that it had a heart."
Comments
Post a Comment