Naked Clams to open a new sector in sustainable nutritious food production | npj Sustainable Agriculture - Nature.com
Abstract
The global population urgently requires alternative food sources that provide the micronutrient-rich profile of meat and fish but with lower environmental cost. We present a solution in the form of 'Naked Clams' (teredinids/shipworms) - a seldom researched group of bivalves, that feature tiny shells and live in and feed on wood, turning it into protein and essential nutrients. We report the first pilot system for Naked Clam aquaculture, the first nutritional profile and feeding efficacy assessment, and demonstrate value offered by microencapsulated feeds in fortifying Naked Clams. Naked Clams were rich in nutrients including vitamin B12 and monounsaturated fatty acids, and shared the high protein content of conventional bivalves such as blue mussels (Mytilus edulis). Microencapsulated algal feeds enriched the Naked Clams with essential PUFAs including EPA and DHA, with potential for further tailoring. Additional work is required, but this study represents a gateway to a new form of sustainable food production.
Similar content being viewed by others
Microencapsulated algal feeds as a sustainable replacement diet for broodstock in commercial bivalve aquaculture
Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable

A comprehensive evaluation of the potential of semiterrestrial isopods, Ligia exotica, as a new animal food
Introduction
The United Nations warns that efforts to curb greenhouse-gas emissions and the impacts of global warming will fall significantly short without drastic changes in global land use, agriculture and human diets1. Increased adoption of 'Blue Foods', foods sourced from aquatic and marine environments, can make up a vital component of this change2. These foods are already important for the economies, livelihoods, and public health of many nations, being nutrient rich3 and typically with a lower carbon, land, and water footprint than most terrestrial meats4. Intensive and controlled or 'urban' aquaculture comprises a lucrative and growing sector, promising faster growth, reduced costs, improved food safety and sustainability5,6,7. Bivalves, species like mussels and clams, are one such 'Blue Food' with outstanding potential8. Bivalves are rich in protein, essential fatty acids and key micronutrients as well as having a lower environmental impact than other sources of protein8,9,10. However, conventional bivalve farming has limitations making the sector financially less attractive than other more profitable but less sustainable sectors such as salmon farming11,12. These problems include habitat degradation and historical over-exploitation13, food safety in increasingly polluted open marine environments9,10, aquaculture and displacement of native species14, slow growth rates compared with fish10, infection and disease in both wild-caught and hatchery grown animals15,16, costly processing, transport and storage10,12. Manufacturer, retail and consumer interest in bivalves is also weak compared to finfish and crustacean aquaculture sectors10,12,17. Lastly, slow growth rates in some bivalves increases costs therefore limiting commercial viability10,18,19.
Teredinids are a seldom researched group of bivalves in the context of aquaculture, yet may offer the answer to providing intensive, rapid, and sustainable growth of nutrient-rich bivalve protein20. Teredinids are the world's fastest growing bivalves and can grow an order of magnitude faster than other bivalves. For example, Teredo navalis, the species of aquaculture potential used in our study, can grow at 1.5–2 mm per day21,22, far outstripping conventional 'large-shelled' bivalves such as mussels, which typically grow at 0.1–0.2 mm per day23,24. Teredinids are unique in that they live in and feed on wood. As such, they do not build large protective shells, but have a tiny, highly-adapted shell for drilling, that only covers the anterior end of the body20,22. Their wood-boring nature formed the foundation of sustainable, low environmental impact early 'farming' of teredinids by Australian Aboriginals as a food source25. Today teredinids are wild-harvested and consumed in regions of Oceania and Southeast Asia, and are considered a delicacy and renowned for their health benefits26. In the Philippines, they are called 'tamilok'27, harvested from dead mangrove trees, and sold in local markets raw and dipped in salt, chilli and vinegar20. In Thailand, they are known as priyang talay, and eaten in curries or braised with fish paste and bananas in a stew20. We note that teredinids are commonly referred to as shipworms, due to their historical legacy of destroying unprotected wooden ships28. However, in the interest of boosting the public image of this under-utilised seafood species and providing a more appetising name, we refer to all teredinids as 'Naked Clams' based on their tiny shells and exposed bodies. There is a rich precedent for rebranding seafood species for palatability and marketability – for example, 'malabar blood snappers', 'rock crabs' and 'slimeheads', are now marketed as 'scarlet snapper', 'peekytoe crabs' and 'orange roughy' respectively29.
A Naked Clam aquaculture system could offer a new opportunity for rapid production of sustainable bivalve meat20. A production system could effectively turn sustainably grown wood from trees into protein for people to eat, supporting circular economy opportunities using recycled wood or wood destined for landfill30,31. Naked Clams could offer reduced processing costs as they do not require shucking10. There is also the potential for enclosed production systems in indoor environments with tailored feed that could optimise growth, quality, nutritional profile and palatability as well as eliminate water quality and food safety concerns20. Further opportunities include installation of production systems alongside other coastal industries such as wind farms, seaweed farms, or located close to the consumers in areas for regeneration and renewal, or even in shipping containers as part of a modular farming system. Moreover, the potential economic opportunity could be great. As one example, UK consumers purchased £8.7 billion of seafood in 201932; hypothetically if a new Naked Clam sector emerged and grew to just 5% the size of this, it would be worth over £400 million. There is already evidence of fast potential Naked Clam animal growth, established markets in Southeast Asia, and purported health benefits. Yet, scaled Naked Clam aquaculture has never yet been attempted.
The underpinning requirement for any form of Naked Clam aquaculture is an understanding of the most effective way to grow and feed them in a scalable aquaculture setting, and critically, an understanding of their nutritional profile. Regarding feeding preferences, the endosymbiotic bacterial microbiome in the gill allows Naked Clams to extract carbohydrates as an energy source and protein for growth from the cellulose, hemicellulose and lignin in wood30,33,34. As filter feeders, it is also thought that the Naked Clams can get nitrogen and carbon from suspended organic matter, such as plankton, within water around the wood35,36. Yet the relative role and importance of wood versus suspended material (such as plankton or dissolved organic material) remains uncertain, with some studies indicating suspended matter is primary and essential36, and others that suggest wood alone is sufficient37. There is a need to understand whether including suspended feed in aquaculture would add additional value to the Naked Clam biomass and nutrient profile. We note, no formal nutritional or micro-nutritional profile of Naked Clams has ever been published in reputable literature20. In the Philippines and Thailand where the clams are consumed, they are renowned for excellent nutritional properties26,38. However, the nutritional profile of Naked Clams on measures comparable to other bivalves such as mussels, as well as levels of key fatty acids and micronutrients, remains unknown. Approaches to Naked Clam aquaculture could be misinformed unless knowledge gaps around their nutritional profile and feeding preferences are addressed.
This study aimed to establish a firm research foundation for Naked Clam aquaculture. We provide the first demonstration of a modular Naked Clam aquaculture system, and the first assimilation efficacy of Naked Clams under different dietary regimes – a crucial step in determining whether fortification (i.e. delivery of additional nutrients beneficial to Naked Clam or human health) is possible using a supplemental suspended feed source (i.e. microencapsulated feed). Importantly, our analysis of faecal production under different dietary regimes provides crucial insight into a controversy around the primary source of nutrition for these animals. In addition, we performed the first formal nutritional profile of a Naked Clam, including fatty acid profile, B12, carbohydrate, fat, and protein content across feeding regimes. This analysis was also the first use of a novel B12 profiling methodology and second trial of a lipid analysis methodology, both developed specifically for bivalve tissue, with high applicability to the aquaculture sector and food regulatory bodies. Together, these data demonstrate a novel aquaculture system that offers scalable and sustainable opportunities for rapid production of low environmental impact bivalve meat in an enclosed production system that eliminates water quality and food safety concerns.
Results
Feeding efficacy
The pilot Naked Clam modular aquaculture system we developed is shown in Fig. 1, and comprises wooden panels in static (non-circulating) aerated aquaria. It was effective in allowing us to assess the relative assimilation efficacy of different feed types. This included a microencapsulated feed which consisted of an algal formulation surrounded by a waxy coating (see Methods). Faecal count analyses revealed that the mean faecal weight from Naked Clams fed on the wood + microcapsule diet (0.26 ± 0.05 (SE) mg) was significantly greater than that from Naked Clams fed on wood only (0.11 ± 0.01 (SE) mg) or wood + Shellfish Diet 1800 (0.13 ± 0.01 (SE) mg) (ANOVA, F(2,6) = 6.27, p < 0.05; Tukey's HSD p < 0.05) (Fig. 2a). Faecal production rates were also significantly lower in Naked Clams fed on wood + microcapsules (15.0 ± 1.5 pellet individual−1 day−1) compared to those fed on wood + Shellfish Diet 1800 (49.0 ± 9.0 pellet individual−1 day−1) (ANOVA, F(2,6) = 10.71, p < 0.05; Tukey's HSD p < 0.01) (Fig. 2b). There was however no significant difference in faecal weight per day between the diet types (ANOVA, F(2,6) = 2.02, p > 0.05) (Fig. 2c). Combined, these three results indicate that whilst there was no overall difference in assimilation rate between diet types (shown by the same faecal weight per day), the retention time for microencapsulated feed in the gut was longer, indicated by the heavier faecal pellet weight and lower production rate from Naked Clams fed on the microencapsulated feed versus those fed on the Shellfish Diet.
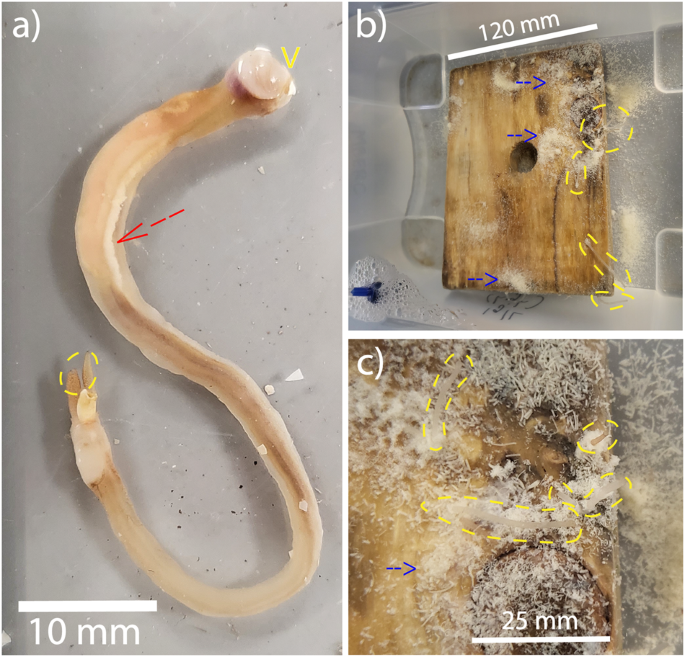
An 82 mm Teredo navalis individual, removed from the wood, is shown in (a). Most of the white meat extends beyond the valves as indicated by a yellow V in (a) – hence the name 'Naked Clams'. Naked Clams can be grown in wood using a simple, modular aquaculture system as shown in (b). The siphons of Naked Clams are the only structures that extend beyond the wooden burrows, and are outlined by yellow dashed lines and magnified in (c). Note the fragments of waste wood dust (frass) covering the wooden panel, shown by the blue arrows, indicating active feeding and growth on wood. This, together with the ripe gonad shown by the red arrow in (a), demonstrates the efficacy of our modular aquaculture system in rearing healthy animals.
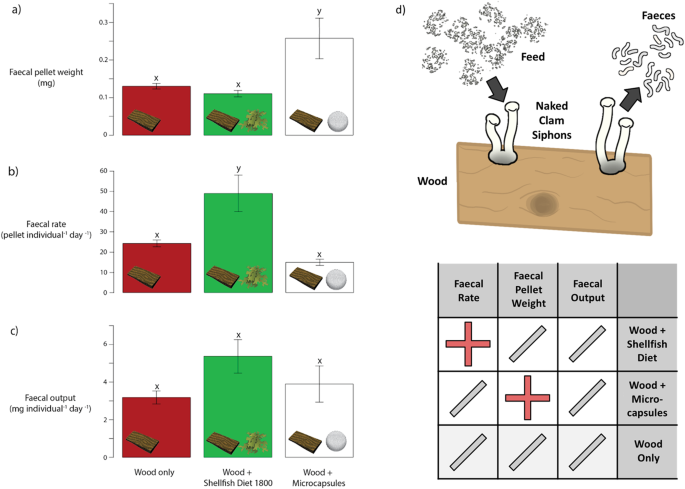
Faecal pellet weight is shown in (a), faecal production rate in (b), and faecal output in (c). Error bars represent standard error of the mean. Letters x and y indicate post-hoc test outcomes, where shared letters indicate no significant difference between groups. A schematic of the feeding assimilation experimental design and efficacy data is shown in (d), with the '+' indicating an increase, and '/' indicating no change.
The Scanning Electron Microscopy - Energy Dispersive Spectroscopy (SEM-EDS) data shows that Naked Clams supplemented with microcapsules display clear differences in faecal material (frass), both compositionally and elementally, compared with control animals fed on a diet solely composed of wood (Fig. 3). SEM images show animals feeding only on wood produced uniform shredded wood fragments in their waste (Fig. 3a), compared with frass that was larger and clumpier in animals enriched with a microcapsule feed (Fig. 3b). This corroborates the findings of our feed assimilation efficacy trials, where animals with a microencapsulated supplemented diet produced heavier faecal pellets (Fig. 2a) and no noticeable pseudofaeces. Subsequent EDS analysis of faecal material from these microcapsule supplemented animals displayed approximately 5× the amount of calcium with minor amounts of silica and sulphur also detected (Fig. 3c–f).
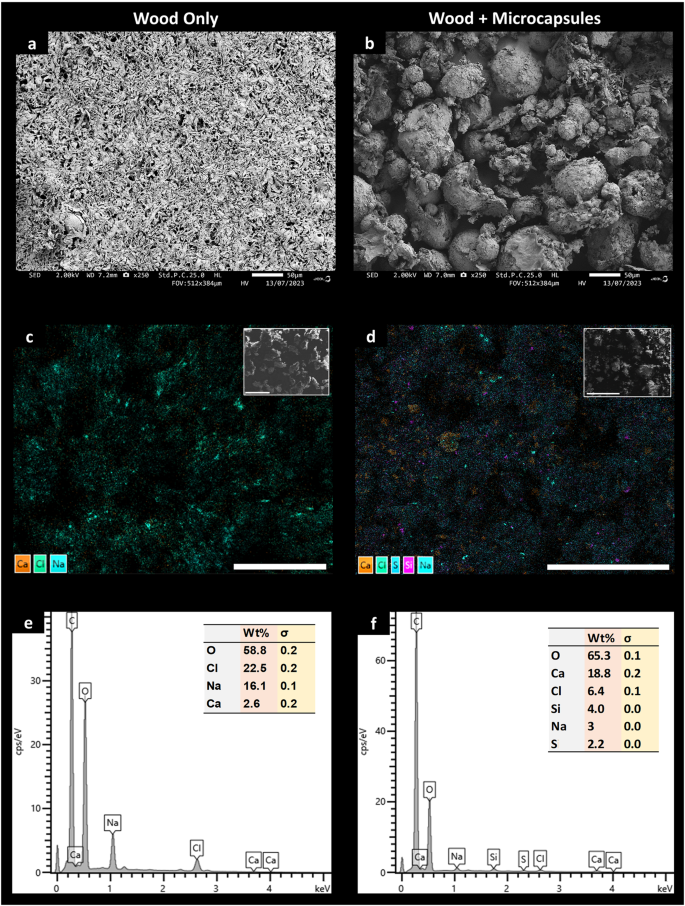
SEM images of Naked Clam faeces at 250× magnification (scale bar = 50 µm) for animal feeding on wood only (a) and wood plus microcapsules (b). Elemental mapping overlay image of faeces for animal feeding on wood only (c) and wood plus microcapsules (d), with raw SEM image displayed in the top right corner. EDS elemental spectra from (c) and (d), shown in (e) and (f) respectively.
Nutritional profile
We were successful in performing the first formal nutritional profile of a Naked Clam (T. navalis). The fatty acid profile, carbohydrate, and protein content, as well as the organic micronutrient vitamin B12 (hereafter B12), was compared across our three feeding regimes, and also against that of the commercially farmed blue mussel (Mytlius edulis, referred to as blue mussels hereon). This analysis was also the first to determine the B12 content of Naked Clams, and a second trial of a lipid analysis methodology developed specifically for bivalve tissue (de-coagulation of bivalve tissues prior to lipid extraction).
Basic biochemical composition analyses revealed that Naked Clams had a higher ash content than blue mussels, and hence lower levels of the metabolite groups protein, fat, and carbohydrate (Fig. 4). Proteins were the dominant metabolite group in both Naked Clams and mussels. Importantly, supplementation with the microencapsulated feed led to a significantly greater protein content than feeding on wood alone, with values of 29% and 22.5% per unit dry weight (DW) (ANOVA, F(1,22) = 14.04, p < 0.05; Tukey–Kramer's HSD, p < 0.05). Carbohydrate content ranged between 16–19% w/w, while lipid content was 3.5–3.8% w/w.
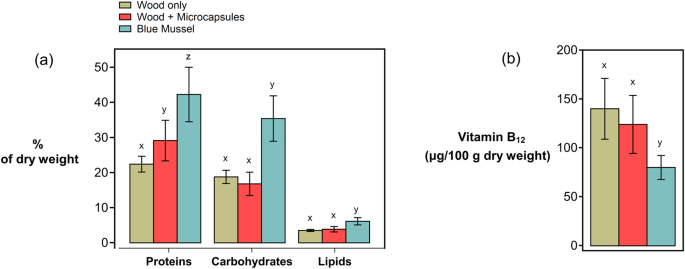
a Protein, carbohydrate, lipid, and (b) vitamin B12 content (expressed as a fraction of total dry weight) of Naked Clams fed with wood only (brown), Naked Clams fed with wood + microcapsules (red), and commercially farmed blue mussels (Mytilus edulis) (blue). Data are expressed as mean +/− standard deviation (SD) of technical replicates (n = 12 for Naked Clams, n = 4 for blue mussels). Letters x, y, and z indicate post-hoc test outcomes, where shared letters indicate no significant difference between groups.
Analyses revealed that Naked Clams had significantly higher levels of B12 than blue mussels (Fig. 4). The B12 content of Naked Clams fed on wood + microcapsules was 142 ± 9 (SE) µg B12 per 100 g DW, significantly greater than that of blue mussels at 81 ± 9 (SE) µg per 100 g DW (ANOVA, F(1,13) = 9.87, p < 0.01; Tukey–Kramer's HSD, p < 0.01). There was however no significant difference in B12 levels between Naked Clams fed on wood only or wood + microcapsules; supplementation with microencapsulated feed did not positively influence the B12 content.
Fatty acid composition assessments revealed that Naked Clams contained a greater content of long chain saturated fatty acids than blue mussels, and that supplementation with microencapsulated feed increases the content of polyunsaturated fatty acids (PUFAs) EPA (Eicosapentaenoic acid, 20:5(n-3)) and DHA (Docosahexaenoic acid, 22:6(n-3)) (Fig. 5). Mass spectrometry revealed that in Naked Clams reared on either wood only or wood + microcapsules, the saturated FAMEs stearic acid (C18:0) and palmitic acid (C16:0) were dominant, followed by the monounsaturated FAMEs oleic acid (C18:1) and palmitoleic acid (C16:1) in high abundance (Fig. 5a). Supplementation of the wood only diet with microencapsulated feed appeared to have an impact on the polyunsaturated acid distribution and content (Fig. 5a). Specifically, while EPA only accounted for 0.8% of the total FAMEs and DHA was not detectable in Naked Clams grown solely on wood, whereas the respective percentages of EPA and DHA in Naked Clams supplemented with microencapsulated were 1.8% and 1.6% (Fig. 5b). Overall, the total polyunsaturated fatty acid content of Naked Clams supplemented with microencapsulated feed was increased by 75.8% compared to wood only feed (ANOVA, F(1,22) = 6.83, p < 0.05; Tukey–Kramer's HSD, p < 0.05). The fatty acid profile of Naked Clams grown in this study differed compared to commercially produced blue mussel tissues tested. The dominant fatty acid of the blue mussel tested was arachidonic acid (C20:4n6, 27%) (Fig. 5a), while EPA, DHA accounted for 1.7% and 10.2% of the total FAMEs, respectively. Although EPA levels were similar to Naked Clams supplemented with microcapsules, DHA and total polyunsaturated FAME content (43.9%) exceeded that of Naked Clams.
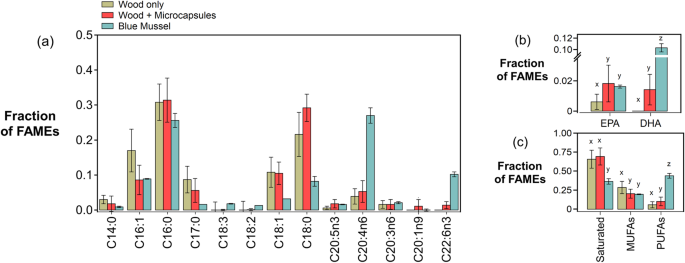
a Fatty acid methyl ester (FAME) composition (expressed as a fraction of total FAMEs) of Naked Clams fed with wood only (brown), Naked Clams fed with wood + microcapsules (red), and commercially farmed blue mussels (Mytilus edulis) (blue). b EPA (Eicosapentaenoic acid, 20:5(n-3)), DHA (Docosahexaenoic acid, 22:6(n-3)), (c) total saturated, mono-unsaturated (MUFAs) and poly-unsaturated fatty acids (PUFAs) are highlighted. Data are expressed as mean +/− SD of technical replicates (n = 12 for Naked Clams, n = 3 for blue mussels). Letters x, y, and z indicate post-hoc test outcomes, where shared letters indicate no significant difference between groups. Full (empirical and chemical) names of fatty acids are shown in Table S1.
Discussion
This study provided the first demonstration of a pilot Naked Clam aquaculture system, and revealed that Naked Clams are naturally rich in nutrients key to human health including protein, vitamin B12, and monounsaturated fats such as oleic acid. We also revealed an important opportunity for nutritional supplementation, demonstrating how Naked Clams can be fortified with additional nutrients using microencapsulated feeds, in this case leading to elevated levels of the essential PUFAs EPA and DHA. The study represents a gateway into a new form of sustainable food production that allows the conversion of wood into a protein and nutrient rich human foodstuff.
We piloted a simple and effective system for the controlled husbandry of Naked Clams in an indoor environment (Fig. 1). The system does not require any complex feeding apparatus or flow-through water exchange systems, and supports active growth of healthy Naked Clams. It provides a foundation for the development of a scaled framework for commercial aquaculture, although further research and development will be required to meet this goal. For example, there is the opportunity for additional growth condition optimisation (e.g. salinity, temperature, wood species substrate), and there is potential to alter the mechanism by which supplemental feed such as microcapsules is delivered to Naked Clams in the aquaria to increase feeding efficiency. While we demonstrate the simplicity, efficacy and value of Naked Clam aquaculture, future studies should optimise growth rates and feeding protocols, which are essential for the commercial application of this novel aquaculture system.
The feeding efficacy studies demonstrated that Naked Clams could successfully digest supplemental suspended feeds, and represents the first time that feeding rates between xlyothophy (wood-feeding) and filter-feeding have been formally assessed in these animals (Fig. 2). By demonstrating that Teredo navalis could grow solely on a diet of wood (Fig. 1), and that feeding rates do not change significantly between xlyotrophy and filter-feeding, we address a controversy in the literature on the source of nutrition for this species37 and show that filter-feeding is an important component of the diet but not the primary source as previously suggested36. Further studies are required to determine the feeding ratios between xylotrophy and filter-feeding in other Naked Clams species. For example, the rock-eating Lithoredo abatanica is likely far more reliant upon filter- feeding39,40, and the giant Kuphus polythalamius (the world's longest bivalve), which inhabits both wood and marine sediments41, is thought to derive nutrition solely from a chemoautotrophic, sulphur-oxidising symbiosis42,43. The longer retention time of the microencapsulated feeds in the Naked Clam gut, relative to liquid algal feed (Shellfish Diet 1800), suggest a greater timeframe for nutrient absorption, indicating that Naked Clams are able to extract more nutrients from microcapsules than from regular algal feed. This is corroborated by the presence of degraded microcapsule matter in the supplement-fed Naked Clam faeces, and the larger, clumpier faecal material that is elementally enriched compared with animals on a wood-only diet (Fig. 3). The presence of degraded microcapsule matter in the Naked Clam faeces, shown by the SEM images and EDS analysis, are further proof of breakdown and absorption of the microcapsules. There is still scope for further work to confirm this analysis, for example a nutritional profile of Naked Clam faeces may allow the quantitative assessment of what proportion of the feed ingested by Naked Clams is fully broken down, and allow us to take further steps in the formulation of Naked Clam feeds to optimise this.
Nutritional analyses revealed that Naked Clams are naturally rich in B12, alongside the monounsaturated fats oleic acid (C18:1) and palmitoleic acid (C16:1). B12 levels in Naked Clams were nearly twice that of B12 levels in blue mussels, at a concentration of 140 µg per 100 g dry weight. The abundance of B12 is a key nutritional selling point of Naked Clams - B12 is made only by certain bacteria44,45, and plants cannot make this vitamin. As such increasing dietary trends towards plant-based diets is resulting in increased occurrence of B12 deficiency or insufficiency44. A serving of just 10 g (dry weight) of Naked Clams per week would meet an individual's entire B12 requirements. We note that the B12 levels of blue mussels analysed in our study fall in line with those published in the literature46. Of further importance, levels of oleic acid in Naked Clams were over 3 times that of the levels in blue mussels. Oleic acid, a monounsaturated fat also found in olive oil, has been repeatedly shown to offer various health benefits, including improvements to cholesterol levels, blood pressure and inflammation, along with decreased heart disease risk and the potential to improve mood and cognition47. The nutritional analyses also revealed that Naked Clams had a higher ash content than blue mussels. This ash, or inorganic mineral content left after analysis, is likely explained by the inclusion of calcareous structures (shells and pallets) and the presence of wood in the guts of Naked Clams. The nutritional results from our study provide a strong foundation to support Naked Clams as a food for mass-market consumption.
The nutritional analyses also emphasised the powerful potential of supplemental feeds, such as microcapsules, to further optimise the biochemical profile of Naked Clams. The microcapsules we used were highly effective in boosting PUFA content; total polyunsaturated fatty acid content of Naked Clams supplemented with microencapsulated feed was increased by 76% compared to wood only feed, and brought EPA levels in line with those of conventional blue mussels. This is explained by the high PUFA content of the microcapsules themselves, which shared a similar formulation to those used in Willer et al. 202019. It is well established that consumption of PUFAs including EPA can help to reduce inflammation, triglyceride levels, and cardiovascular disease risk factors in humans48. Fish currently stands as the primary means of obtaining these nutrients via food, with the UK government recommendation being an intake of 280 g a week49, and 2020 consumption currently standing at around 160 g per week50. Increasing consumption to recommended values with our current production systems would be environmentally unsustainable if adopted by the UK and other nations51, and Naked Clam meat could offer a means by which to sustainably meet this intake. The waxy encapsulant of the microcapsules may also contribute to the higher lipid content in the Naked Clams. We do note that DHA levels in Naked Clams supplemented with microencapsulated feed were still slightly lower than those in blue mussels, and that we also did not run a nutritional profile on Naked Clams fed on suspended algal feed, and hence don't know the performance of algal feed versus microcapsules. This highlights the outstanding need for further optimisation of supplemental feeds for Naked Clams, to identify which formulations and delivery approaches represent the best way to optimise their nutritional profiles. The microcapsule itself offers a powerful delivery vehicle, and as demonstrated in other studies18,19,52,53 may offer a means by which to fortify Naked Clams with specific nutrients lacking in a specific population or socio-demographic sector53.
Beyond the steps suggested above and the continued development of the Naked Clam aquaculture system, there is still a requirement for engagement across the value chain in order to realise the potential benefits of Naked Clam consumption to human health and environmental sustainability. Attracting investment from aquaculture corporations or startups to engage with and adopt the Naked Clam aquaculture system will be key in this regard. Sustainable and reliable supplies of wood feedstock also need to be carefully selected, and cold-chain solutions for the effective harvest, distribution, and processing of Naked Clam meat. Engagement with food standards authorities will be required; while Naked Clams are sold as food in Asia they are not yet in Western economies. We also suggest that at this point in time, development focuses on indoor modular aquaculture systems as piloted in our study, as opposed to open water aquaculture systems, where additional care is required given the economic damage teredinids cause to submerged wooden coastal structures54. At the food manufacturer and consumer end, there is a critical requirement to identify the most effective means to turn Naked Clam meat into a mass market product. For example, will Naked Clams be best sold as a fresh meat item like in southeast Asia, or in simply processed formats (e.g. minced, purees), more complex formats (e.g. supplemental dry powders), highly advanced formats (e.g. textured flavoured extruded proteins), or a combination of the previous? Effective consumer research and marketing will be pivotal to the success of attempts in this field.
Overall, this study has provided the experimental foundation for a new form of sustainable food production that could turn wood into a protein and nutrient source for mass market human consumption. Naked Clams are hardy, grow exceptionally fast, and can be produced in static saltwater systems. Here we have demonstrated a viable Naked Clam aquaculture system, assessed feeding efficacy, revealed a naturally rich nutritional profile, and outlined an opportunity for further fortification with supplemental feeds. There is an opportunity here to build a completely new aquaculture sector and open up a wealth of avenues for sustainable food production and consumption.
Methods
Sample collection
Teredo navalis specimens were sourced from north-eastern US coastal waters. To obtain samples for the experiments, two Eastern pine (Pinus strobus) panels of 200 × 120 × 20 mm were placed in Gloucester Harbour, MA, USA on 4/5/2021, to allow settlement of Teredo navalis larvae. These panels were retrieved on 13/3/22. Larval settlement will have taken place during the warmer summer months of July, August and September in 2021, and so the T. navalis were approximately 9-month-old adults at the point of collection. Blue mussels (Mytilus edulis) for the nutritional analysis were commercially farmed and were sourced from Shetland, Scotland. These mussels were rope-grown, fed on wild marine phytoplankton, and of two years age and market size at the point of harvest in February 2023.
Microencapsulated feed manufacture and profiling
Lipid-walled microcapsules containing 30% powdered Schizochytrium algae by weight were manufactured under patent by BioBullets (BioBullets Ltd, Cambridge, UK). To manufacture the microcapsules a premix slurry containing a waxy encapsulant with antibacterial properties and powdered algae were prepared under conditions of controlled shear. The slurry was pumped into an ultrasonic atomizing nozzle at the top of a cooling chamber. The atomized particles formed near-perfect spheres as they cooled and fell to the chamber base. Further particle cooling was achieved with an air-conveying system before discharge via cyclone to a fluid bed processor. The encapsulated feed was then coated with a proprietary non-ionic surfactant to aid dispersion in water. Further cooling in the fluid bed removed all heat of crystallization from the microcapsules before packaging. All components of the formulation were food grade. The physical characteristics of the microcapsules were quantified using a Malvern Mastersizer particle size analyser. The microcapsules had a mean diameter of 46.6 µm, with a DV(10) of 17.7 µm and DV(90) of 104 µm. The microcapsules had spherical shape, near neutral buoyancy, and were tailored for bivalve consumption and growth9,18,19,52,53.
Laboratory conditions
Laboratory experiments took place in the Davy Building aquarium, University of Plymouth, England, in aerated tanks in temperature-controlled rooms maintained at 15 °C.
For the feed assimilation efficacy experiments, three wooden panels containing T. navalis specimens were placed in three separate 1.6 litre tanks (MW, SDW, and W) from 21/11/2022 till 09/12/2022 (Fig. 1). The panels in tanks MW and SDW had 7 pairs of siphons each, and the panel in tank W 5 pairs of siphons, indicating the presence of 7, 7 and 5 individuals respectively. The panels were exposed to three different dietary regimes – microcapsules + wood (MW), Shellfish Diet 1800 + wood (SDW), and wood only (W). Shellfish Diet 1800 is an algal concentrate blend (Shellfish Diet 1800, Reed Mariculture, California, USA). To ensure valid observations, feeding rates were optimised to minimise pseudofaeces expulsion, given faecal expulsion was the measure of feeding rate in this study. Bivalves are known to expel indigestible particulates in the form of pseudofaeces in high concentrations of particulate matter, and studies on pseudofaeces production have found that particulate concentrations around 2 mg/L show minimal production of pseudofaeces within oysters55,56. This correlated with using a recommended feeding rate of 3% dw (dry weight) feed per dw bivalve per day57. Feeding took place at 09:00 every Monday through Friday, and at 15:00 every Monday through Thursday. Feeding did not occur at 15:00 on Friday as that was when faeces were both counted and collected for weighing. Two half feeds were used in these experiments to avoid the production of pseudofaeces, which could have risen from oversaturation.
For the nutritional profiling experiments, the two wooden panels containing T. navalis juveniles were placed into two separate 10 litre tanks (MW and W) on 09/05/2022 (Fig. 1). The panel in tank MW had 40 pairs of siphons and the panel in tank W 28 pairs of siphons, indicating the presence of 40 and 28 juveniles respectively. The panels were exposed to two different dietary regimes – microcapsules + wood (MW), and wood only (W). Initial juvenile dry weight was measured as 0.069 g, and used to calculate feeding ration for microcapsules at a rate of 3% dry weight (dw) capsule per approx dw bivalve per day57. Microcapsules were fed at this rate via a three times weekly feed, with care taken to fully disperse the microcapsules. Juveniles from the MW tank were harvested on 30/06/2022, and juveniles from the W tank were harvested on 02/02/2023.
Feed assimilation efficacy
Faecal rate was observed through two methods, daily digital recording and weekly visual counting. Recording was performed after the 3PM feeding every Monday through Friday using GoPro HERO 11 cameras. For each sample, 10 mins of recording on the siphons was performed for each visible individual in each tank. Recordings were viewed later, and a daily rate was calculated based on the number of faecal pellets seen expelled from the syphons within the timeframe.
The weekly faecal count was observed every Friday at 15:00. High quality top-down pictures of the containers were taken using the GoPros, capturing all the faecal pellets within the container. If one picture of the entire container was not sufficient, smaller pictures were taken instead of each quarter of the tanks. Faecal pellets were counted using visual identification assisted with Microsoft Paint (2023 Version, Microsoft, California, USA), which was used to section off parts of the image and mark counted pellets in an obvious colour, noting the number of pellets counted in each segment. Once these segments were accurately counted and added up, the average rate of each individual was calculated, and the total number would be used for calculating average pellet weight.
Faecal weight was also gathered at 15:00 every F...
Comments
Post a Comment